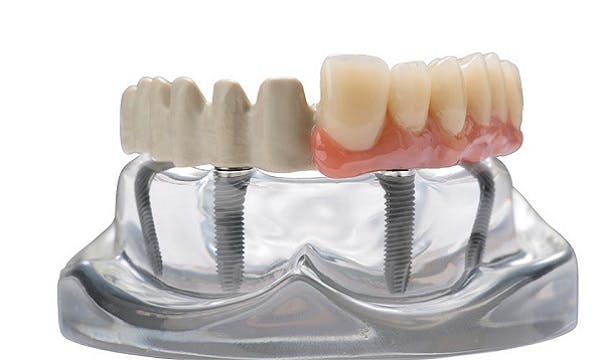
High performance polymer dental prosthetic device can potentially enable improved patient quality of life1 and efficiencies compared to metal.
Thornton Cleveleys (UK) – A major innovation in dental prosthetics for comfort, durability and precision, the JUVORA™ dental disc, has received the US Food and Drug Administration 510(k) clearance for expanded indications. Indications for use in the United States now include the manufacture of full and partial removable dentures and implant overdentures, as well as copings, substructures (cemented or uncemented), and frameworks for permanent and transitional anterior or posterior crowns and bridgework. JUVORA implant prosthetic frameworks can be tailor-made to patients´ specific needs, using CAD/CAM workflows, and result in reduced treatment times and better treatment outcomes.
Following its successful CE mark approval in Europe and introduction onto the European dental market in 2012, the JUVORA dental disc received initial FDA 510(k) clearance for purchase and use throughout the US in 2014. With this latest clearance, the FDA has cleared a CAD/CAM device made of PEEK polymer for the manufacture of long-term implant-borne prosthetic frameworks, compatible for use with fixed rehabilitation systems including the ALL-ON-42 treatment concept and, the STRAUMANN3 PRO ARCH system.
This proven alternative to metal prosthetic devices is made from PEEK-OPTIMA™ high performance polymer. The biomaterial with more than 15 years of proven clinical efficacy is sourced exclusively from Juvora´s parent company Invibio Ltd. It offers a superior combination of strength, natural bone-like flex and toughness, with more than five million PEEK-OPTIMA-based devices now implanted worldwide.
Significant improvement for dental prosthetic patients
In addition to receiving endorsements from a growing number of dental labs and dental practitioners, the feedback from patients has been extremely positive. An overwhelming 99% of patients rated JUVORA prosthetics high for comfort and 97% rated them high for overall satisfaction.4
"Patients have told us their JUVORA prosthetics feel like having their natural teeth back. This is not to mention a reduction in weight, and superior comfort through tailored fit because the prosthetic has been made with precision through CAD/CAM workflows", noted Lynne Todd, Head of Invibio Dental and Juvora.
What sets the JUVORA dental disc apart are the outstanding properties of PEEK-OPTIMA™ Natural, the world’s first implantable PEEK polymer which is widely recognized within the spinal interbody fusion market and frequently used. The unique feature-set of the biomaterial, and accordingly the JUVORA dental disc, includes a bone-like modulus which gives the JUVORA implant prosthetic framework the potential to react in a more natural way to the stresses and forces it encounters.5
A new era in the precision design of advanced dental prostheses
“Dentists in the US are now able to offer dental prosthetics that are not only more comfortable than those made from metal, but also more accurately and efficiently designed. JUVORA dental prosthetics can be a compelling alternative to metal for long term fixed as well as removable implant prosthetic applications, such as frameworks and other superstructures – both for the dentist and for their patients," summarised Todd.
Further information about JUVORA can be obtained online at juvoradental.com and at www.youtube.com/user/JUVORADental Further information about the medical uses of PEEK polymer can also be obtained online at www.medicalpeek.org
References
- B. Siewert (2017), PEEK in Dental Prosthetics (PEEK in der zahnärztlichen Prothetik Warum? Wann? Wie?), Podium Presentation, SSO Dental Meeting, 11 February 2017
- The “ALL-ON-4” treatment concept is a trademark and property of Nobel Biocare Services AG
- The “STRAUMANN” name is a trademark of Straumann Holding AG.
- Patient feedback from 92 cases July 2013 – March 2015
- Bone Regeneration Based on Tissue Engineering Conceptions —A 21st Century Perspective Jan Henkel, Maria A. Woodruff, Devakara R. Epari, Roland Steck, VaidaGlatt, Ian C. Dickinson, Peter F. M. Choong, Michael A. Schuetz & Dietmar W. Hutmacher, Bone Research (2013) 1, 216–248