A Non-Metal Alternative for Extremity Applications
A metal-free solution for extremity applications
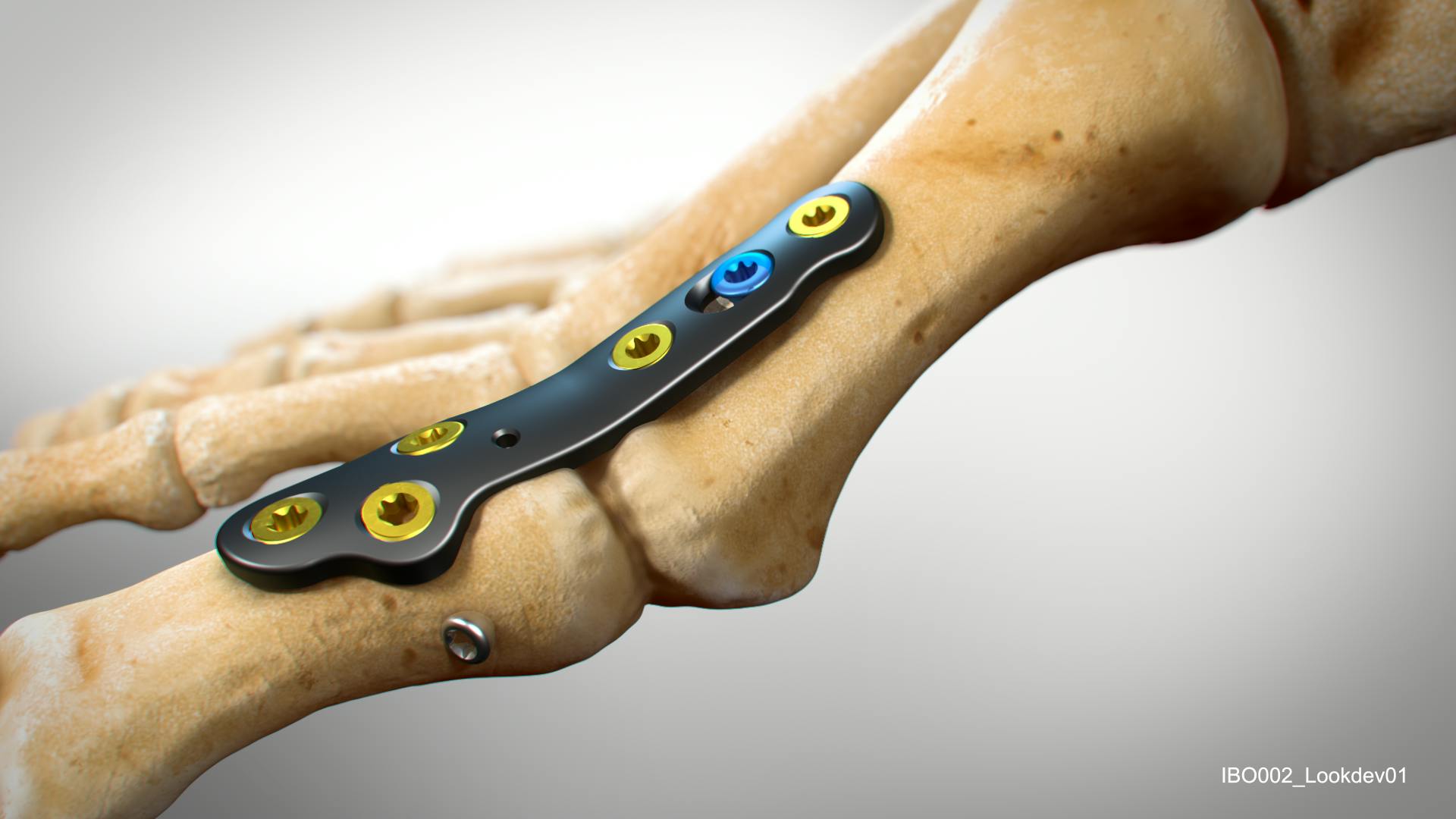

Increasing Adoption of PEEK-based Devices for Hand and Foot Applications
Foot Implants: PEEK Devices for Foot & Ankle Applications
A metal-free solution for extremity applications



Increasing Adoption of PEEK-based Devices for Hand and Foot Applications
Both unfilled PEEK and carbon fiber reinforced PEEK materials have been successfully used in plating applications for the hand and foot.
Demonstrated benefits include:

PEEK-based intramedullary devices can provide similar advantages as metallic designs with potential additional benefits:
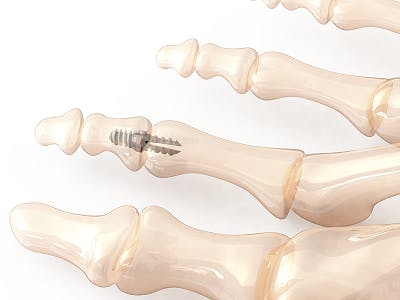
Image: Vector™ Hammertoe Correction System manufactured from PEEK-OPTIMA™ HA Enhanced, courtesy of Nvision Biomedical Technologies, Inc.™

Studies on implanted PEEK spacers for osteotomies in the foot demonstrate high fusion rates and conclude that PEEK offers:
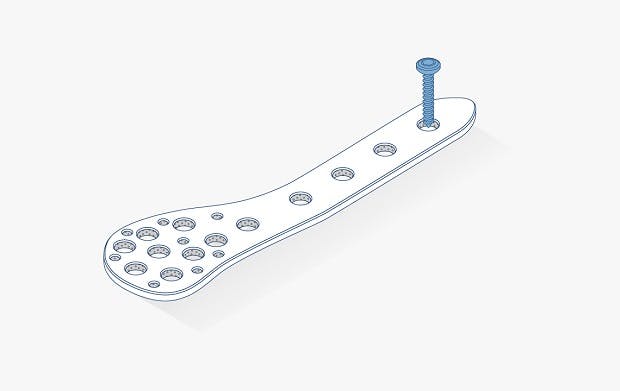
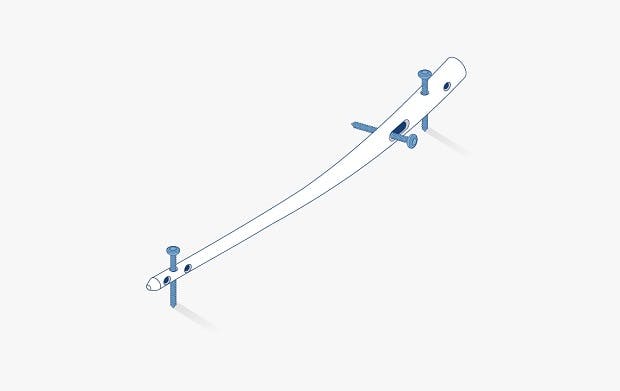
Image: The Trigon® Stand–Alone Wedge Fixation System, courtesy of Nvision Biomedical Technologies, Inc.™ Read more about it here

First FDA clearance for an osteotomy wedge system made of PEEK-OPTIMA™ HA Enhanced

References
1.Rhee PC, Shin AY. The rate of successful four-corner arthrodesis with a locking, dorsal circular polyether-ether-ketone (PEEK-Optima) plate. J Hand Surg Eur. Vol. 2013 Sep;38(7):767-73.
2. Harmer JL, et al. A Midterm Review of Lesser Toe Arthrodesis With an Intramedullary Implant. Foot Ankle Spec. 2017 Oct;10(5):458-464.
3. Walsh WR, et al. Does PEEK/HA Enhance Bone Formation Compared With PEEK in a Sheep Cervical Fusion Model? Clin Orthop Relat Res. 2016 Nov; 474(11): 2364–2372.
4. Study evaluated the in vivo response to PEEK-OPTIMA™ Natural, PEEK-OPTIMA™ HA Enhanced and allograft in a cervical spine fusion model in sheep. Data on file at Invibio. This data has not been correlated with human clinical experience.
5. Cook JJ, Cook EA. (2019). Proximal Interphalangeal Joint (PIPJ) Arthrodesis with a Poly-etheretherketone (PEEK) Implant. In: Cook E, Cook J (eds) Hammertoes.
Springer, Cham. https://doi.org/10.1007/978-3-319-16552-3_11
6. Gomez DN, Eslava S, Federico A, et al. Use of Poly(Ether Ether Ketone) cages in foot and ankle surgery. Foot Ankle Clin. 2012 Sep; 17(3): 449-57.
7. Zaghloul KM, Saied AM, Abouelnas BA, et al. Can polyaryletherketone cage be used to achieve union and maintain correction in anterior calcaneal lengthening
osteotomy for treatment of flexible flatfoot? J Pediatr Orthop B. 2019 Nov;28(6):598-601.
8. Pearson KT. Clinical Case Highlight: Tarsa-Link™ Stand-Alone Wedge Fixation System for flatfoot deformity repair – Evans osteotomy. http://www.
centricmedical.com/tarsalink-case-study-1. Accessed May 28, 2020.
9. 1. https://www.nextremity.com/new-research-indicates-up-to-60-million-americans-suffer-from-hammertoe-foot-deformities/. Accessed May 26, 2020
Trigon® Stand–Alone Wedge Fixation System for Cotton Osteotomies, image courtesy of Nvision Biomedical Technologies, Inc.™. Trigon® is a registered trademark of Nvision Biomedical Technologies, Inc.™
Vector™ Hammertoe Correction System manufactured from PEEK-OPTIMA™ HA Enhanced image courtesy of Nvision Biomedical Technologies, Inc.™ Vector® is a registered trademark of Nvision Biomedical Technologies, Inc.™

From the invention of PEEK over 40 years ago, Victrex has continually pioneered new PAEK-based polymers, materials and solutions that have transformed markets, delivering global impact in the toughest environments.
We bring transformational & sustainable solutions that address world material challenges every day.