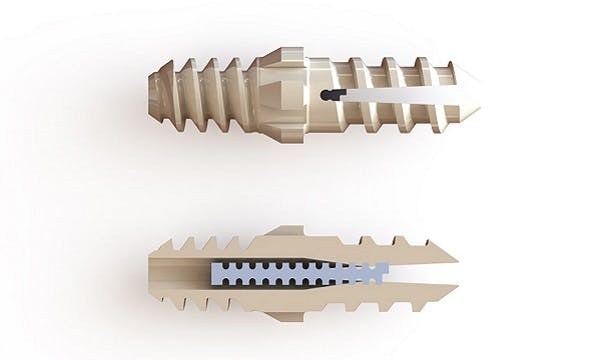
Innovative implant from Nvision corrects hammertoe, and expands product offering to lower extremities
San Antonio, TX (US) / Thornton Cleveleys (UK), March 11, 2019 – The Vector™ Hammertoe Correction System, a bioimplant from Nvision Biomedical Technologies, a medical device and biologics company, has been cleared by the FDA for use in the US. It is the first foot and ankle implant to be made from the advanced, biocompatible PEEK-OPTIMA™ HA Enhanced, a polymer from Invibio Biomaterial Solutions™. It is also the first lower extremity (Proximal Interphalangeal Joint Arthrodesis) implant to use Structural Encoding® to enable the Unique Device Identification (UDI) required by the FDA.
From focusing on spinal implants, Nvision is now expanding its product portfolio to lower extremities – with the goal to improve patient quality of life in this segment. "Hammertoe correction is one of the most common foot and ankle procedures, but we realized there are opportunities to improve outcomes," said Tom Zink, Nvision Senior Vice President of Product Development. "We have seen real patient success with our interbody fusion product line using Invibio’s PEEK-OPTIMA™ Natural polymer. Now with our Vector Hammertoe Correction System, we are utilizing the PEEK-OPTIMA™ HA Enhanced material to translate and expand this innovation in foot and ankle surgeries.”
Strong partnership to transfer bone apposition benefits to lower extremity applications
Nvision has developed the Vector Hammertoe Correction System for Proximal Interphalangeal Joint Arthrodesis to offer innovative features including the potential of early bone ongrowth using PEEK OPTIMA HA Enhanced. In addition, it gives surgeons the ability to correct hammertoe issues with a 100% revisable implant.
Invibio closely partnered with Nvision to develop testing, and optimize the manufacturing process, while providing support regarding regulatory requirements for the FDA 510(k) submission to obtain clearance for the new implant.
The Vector Hammertoe Correction System is also the first lower extremity implant to incorporate Structural Encoding, a patented technology platform licensed from Watershed Idea Foundry that enables the unique identification of medical devices, by means of permanent direct part marking. The technology is able to embed the entire history of the device, and the data can be read by simple X-ray imaging.
"To date, devices made from PEEK-OPTIMA HA Enhanced have been used exclusively for spinal interventions. Nvision's Vector system is the first time we've seen such a device cleared by the US FDA for other than spinal applications," noted John Devine, Medical Business Director, Invibio Biomaterial Solutions. "What we've achieved here, with Nvision and Invibio working as project partners, is a solution for a relatively common but curiously challenging problem, the curled up, calcified toe. The result is a very small device, but one that for many hammertoe patients could literally shape a better future."
PEEK-OPTIMA HA Enhanced provides a potential solution for fusion across the joint, a surgeon's primary goal for hammertoe surgery. It does so because hydroxyapatite (HA) is fully integrated within the matrix of Invibio's PEEK-OPTIMA Natural. This means the HA is integrated, not coated, and available on all surfaces of a finished device. In combination with a modulus of elasticity closely matching that of actual bone, PEEK-OPTIMA HA Enhanced has the potential to promote bone ongrowth and healing. Its radiolucent properties result in artifact-free imaging with the ability to easily monitor the healing process.
Nvision´s new foot and ankle implant enables a standard surgical technique that allows direct drilling and the exact placement of the implant. In doing so, the Vector Hammertoe Correction System offers considerable advantages over a conventional K-wire implant procedure:
- It achieves a press fit between the implant and the bone since the cavity is accurately drilled and controlled.
- The precision instrumentation of the Vector system promotes ease of insertion, further improving the success of the procedure.
- The Structurally Encoded pin, once pressed into position, allows for a greater pull-out force than other PEEK hammertoe implants.
Nvision realized that the small cross sectional area of the intermediary canal makes PEEK-OPTIMA HA Enhanced, with its potential for promoting osteointegration on the implant, the perfect material for hammertoe correction. Fusion is expected to be stronger, and the patient should be satisfied with the outcome.
AAOS 2019 Annual Meeting, March 13-15, Las Vegas, Nevada: Invibio and Nvision to showcase milestones
Driving innovation for the foot and ankle segment, Nvision is currently exploring the development of additional lower extremity devices using PEEK-OPTIMA HA Enhanced. At the AAOS (American Academy of Orthopaedic Surgeons) Annual Meeting - March 13-15, 2019 Nvision will showcase its recent innovations at booth 4861, and Invibio at booth 2829.
For further information about Nvision Biomedical Technologies, visit www.nvisionbiomed.com
For further information about Invibio Biomaterial Solutions, visit www.invibio.com
About Nvision Biomedical Technologies
Nvision is a San Antonio-based medical device and biologics company focused on providing surgeons with implants paired with instrumentation and biologics that simplify and improve surgery procedures to help patients get back to their quality of life. Nvision is committed to developing and manufacturing thoughtfully designed products combined with exceptional service that keep the patient, surgeons, healthcare providers and distribution partners in mind. Nvision is aligned with surgeon thought leaders, key researchers, and development engineers from prestigious institutions to design, test, and bring to market the most innovative concepts. Find out more at www.nvisionbiomed.com
About Invibio Biomaterial Solutions
Invibio, part of the Victrex plc group of companies, is a global leader in providing high-performance biomaterial solutions to medical device manufacturers. The company provides PEEK-OPTIMA™ polymers, advanced technical research and support and manufacturing of components for spine, trauma and orthopaedic and dental medical segments for the development of long-term implantable medical devices. Today, Invibio’s PEEK-OPTIMA™ polymers are used in approximately 9 million implanted devices worldwide.
INVIBIO™, PEEK-OPTIMA™, INVIBIO BIOMATERIAL SOLUTIONS™ are trademarks of Victrex plc or its group companies. All rights reserved. Find out more at www.invibio.com
About Victrex plc
Victrex is an innovative world leader in high performance PAEK/PEEK polymer solutions, focussed on the strategic markets of automotive, aerospace, energy (including manufacturing & engineering), electronics and medical. Every day, millions of people use products and applications, which contain our materials – from smart phones, aeroplanes and cars to oil and gas operations and medical devices. With 40 years’ experience, we develop world leading solutions in PEEK and PAEK-based polymers and selected semi-finished and finished parts which shape future performance for our customers and our markets, and drive value for our shareholders. Find out more at www.victrex.com
Media Contacts
Invibio Biomaterial Solutions media contact
Barbara Pasciak, Marketing Communications Manager, barbara.pasciak@invibio.com,Phone 484-342-6041
Nvision Biomedical Technologies media contact
Bonnie Caver, bonnie@replighthouse.com, Phone 512-217-5397
Victrex media contact
Beate Sauer, PR & Marketing Communications Manager, bsauer@victrex.com, Phone +49 61 92 / 96 49 19
Victrex corporate, financial media or investor enquiries
Andrew Hanson, Director of Investor Relations & Corporate Communications, ahanson@victrex.com, Phone +44 12538 98121
Victrex plc and/or its group companies (Victrex plc) believes that the information contained in this press release is an accurate description of the typical characteristics and/or uses of the product(s) and is based on information that we believe is reliable. However, it is provided for information only. It is not intended to amount to advice on which you should rely and should not be construed as, or used as a substitute for other professional or specialist advice. In particular, it is the customer’s responsibility to thoroughly test the product in each specific application to determine its performance, efficacy, and safety for each end-use product, device or other application. Suggestions of product uses should not be taken as inducements to infringe any particular patent. Mention of a product in this document is not a guarantee of its availability.
Victrex plc reserves the right to modify products, specifications and/or packaging as part of a continuous program of product development. Victrex plc makes no warranties, express or implied, including, without limitation, a warranty of fitness for a particular purpose or of intellectual property non-infringement, including but not limited to patent non-infringement, which are expressly disclaimed, whether express or implied, in fact or by law.
Further, Victrex plc makes no warranty to your customers or agents, and has not authorised anyone to make any representation or warranty other than as provided above. Victrex plc shall in no event be liable for any general, indirect, special, consequential, punitive, incidental or similar damages, or any damages for harm to business, lost profits or lost savings, even if Victrex has been advised of the possibility of such damages regardless of the form of action. The foregoing does not seek to affect our liability in respect of: (i) death or personal injury arising from our negligence; (ii) fraudulent misrepresentation; (iii) product liability or other consumer remedy claims brought by an individual consumer in relation to our products; (iv) nor any other liability which cannot be excluded or limited under applicable law.
Copyright ©2019 Invibio Ltd. INVIBIO™, PEEK-OPTIMA™, INVIBIO BIOMATERIAL SOLUTIONS™ are trademarks of Victrex plc or its group companies. All rights reserved.
You may also be interested in...







