PEEK Biomaterials for 3D Printed Spinal Implants

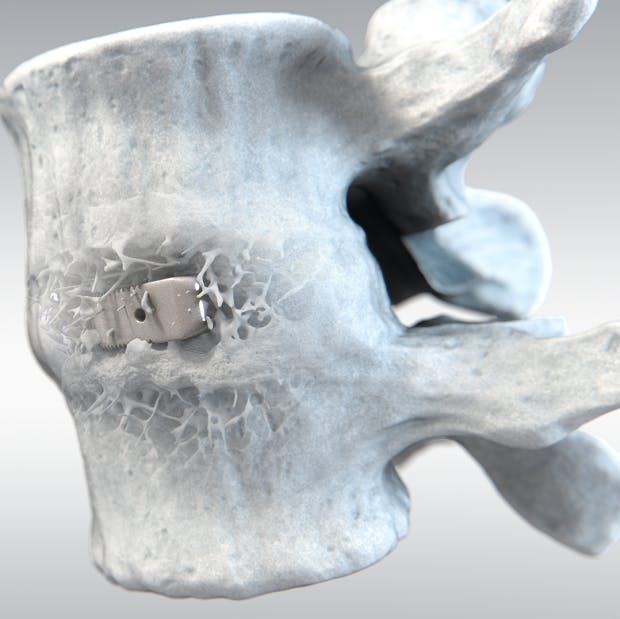
Optimized bone on-growth in spine

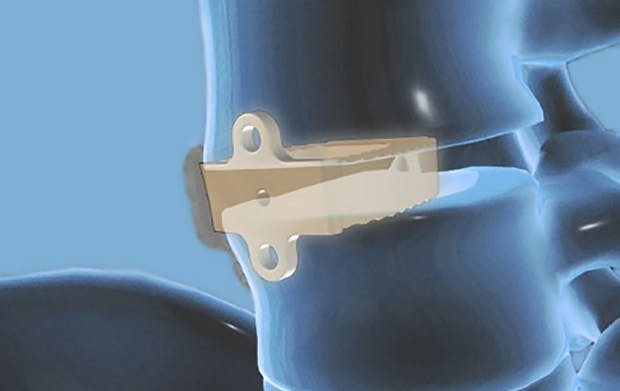


Optimized bone on-growth in spine



Invibio is here to help you every step of the way through the design, regulatory, manufacturing and commercialization process providing unmatched opportunities to bring next generation spine medical devices to market quickly.
Surgeons discuss their first-hand experiences with PEEK-OPTIMATM HA Enhanced polymer devices
Future-proofing with 3D printed implants
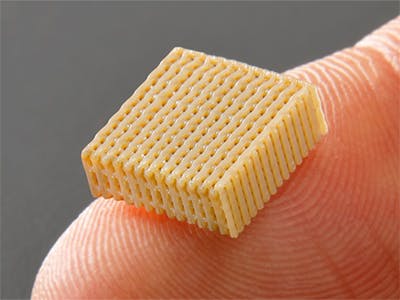
Improved technologies for 3D printing of PEEK-OPTIMATM based polymers can open up a range of possibilities for medical device design engineers. Invibio is working exclusively with Bond3D to offer technology to additive manufacture biocompatible, PEEK functional parts with isotropic tensile strength.

“The best of all worlds”: A surgeon’s clinical experience with the Lucent® XP Expandable Interbody device






References
1. IQVIA, Spinemarket Inc. 2021, Minneapolis, MN.
2. Study evaluated the bone ongrowth of PEEK-OPTIMATM Natural and PEEK-OPTIMATM HA Enhanced in a bone defect model in sheep. Data on file at Invibio. This has not been correlated with human clinical data.
3. Study evaluated the in vivo response to PEEK-OPTIMATM Natural, PEEK-OPTIMATM HA Enhanced and allograft in a cervical spine fusion model in sheep. Data on file at Invibio. This data has not been correlated with human clinical experience.
*Lucent® is a registered trademark of Spinal Elements.

From the invention of PEEK over 40 years ago, Victrex has continually pioneered new PAEK-based polymers, materials and solutions that have transformed markets, delivering global impact in the toughest environments.
We bring transformational & sustainable solutions that address world material challenges every day.