PEEK-based devices for hand and foot applications are experiencing an increase in adoption, with growing clinical evidence demonstrating the benefits PEEK can offer. Invibio’s range of materials from unfilled PEEK to hydroxyapatite (HA) impregnated PEEK and carbon fiber reinforced PEEK can address requirements of numerous hand and foot applications, taking advantage of PEEK’s radiolucency, modulus similar to bone, and biocompatibility.
Hammertoe Devices
Hammertoe deformities are common, affecting 10-20% of the general population in developed countries.1 Kirschner wire (K-wire) fixation is the most common treatment for fixed hammertoe operative correction,2 but newer intramedullary devices are becoming increasingly available to address the weak points of K-wire fixation.3 K-wires carry inherent risks of pin tract infection, migration, and can be cosmetically unappealing.2 Recent comparative studies demonstrate a trend toward increased patient satisfaction,4,5 improved pain relief, better union rates,2,4,5 and decreased rates of deformity recurrence and infection2,4 in patients treated with intramedullary devices of various materials versus K-wires.
Intramedullary devices are designed to be intraosseous with enhanced stabilization during fusion. Features such as threads or ridges can impart rotational stability, and in some designs, enable controlled compression at the arthrodesis site. PEEK is capable of being machined to the same designs as many metallic intramedullary devices and have been cleared for a number of the same indications.
PEEK-based intramedullary devices can provide similar advantages as metallic designs with potential additional benefits. Most notably, the radiolucency of the material can enable an easier assessment of fusion. The modulus of elasticity is very similar to bone, so there is minimal stress shielding at the arthrodesis site reducing risk factors that could disrupt normal bone healing.6 A criticism of metallic intramedullary devices is how to remove them. PEEK-based devices can simplify removal because it can be easier to saw through the PEEK.
In recent years, there has been a trend to improve fixation of PEEK devices for interbody fusions, and this trend is also evident in proximal interphalangeal joint arthrodesis (PIPJ). There were eight FDA 510(k) clearances for unfilled PEEK devices from 2014-20157 followed by a titanium plasma spray coated PEEK device in 2018,8 and most recently, a PEEK-OPTIMA™ HA Enhanced device in 2019.9
The Vector™ Hammertoe Correction System (Nvision Biomedical Technologies) incorporates Invibio’s PEEK-OPTIMA HA Enhanced polymer to take advantage of the material’s osteoconductive properties.

Figure 1 Vector™ Hammertoe Correction System depicts a postoperative radiograph of three hammertoe fusions with the PEEK-OPTIMA HA Enhanced PIPJ fusion devices and one fusion with a K-wire for comparison

Figure 2 PIPJ arthrodesis of 2nd, 3rd, and 4th ray using the Vector™ Hammertoe Correction System manufactured from PEEK-OPTIMA™ HA Enhanced (right). The visible metallic component is a patented tantalum structural encoded identifier for Unique Device Identification (UDI). Images provided courtesy of Nvision Biomedical Technologies. ©Nvision Biomedical Technologies.
Wedge Osteotomy Devices
Continuing with the trend of applying material innovations from the spine to the foot, regulatory cleared devices for interbody fusion have been used off-label for foot applications.10,11 Over time, specifically preconfigured wedges have been developed for lateral column lengthening and medial cuneiform opening wedge osteotomies. Like interbody devices, titanium wedges were followed by the launch of PEEK-based devices.
Studies on implanted PEEK spacers for osteotomies in the foot demonstrate high fusion rates and conclude PEEK is an effective alternative to structural bone grafts,12,13 and may provide advantages in terms of maintenance of correction because there is no risk of resorption of the graft material.14 Like hammertoe devices, a range of material offerings from natural PEEK to titanium plasma spray coated PEEK to PEEK-OPTIMA HA Enhanced polymer (Figure 2) have been cleared to market, with the latter two intending to enhance fixation. The PEEK spacers can be designed as wedges to be used with ancillary fixation or as stand-alone devices with integrated screws. Two recent case studies using stand-alone devices for flatfoot deformity repair conclude successful outcomes to date, with no complaints or return of deformity or pain and each patient returned to competitive athletics.14, 15


Figure 3: Trigon™ HA Stand-Alone Wedge Fixation System (a) Evans lengthening osteotomies (left) and (b) Cotton (opening wedge) osteotomies of the medial cuneiform (right). The system is manufactured from PEEK-OPTIMA™ HA Enhanced. Images provided courtesy of Nvision Biomedical Technologies. ©Nvision Biomedical Technologies.
Plating
Both unfilled PEEK and carbon fiber reinforced PEEK materials have been successfully used in plating applications for the hand and foot.
Hand
Four-corner arthrodesis with scaphoidectomy is a motion-sparing procedure to address scapholunate advanced collapse, the most common pattern of degenerative arthritis in the wrist. Traditional methods of fixation include K-wire, screw, or staple fixation.16 Dorsal circular cups were introduced to promote more rigid fixation allowing early active range of motion (ROM) exercises.17 An all-PEEK locking dorsal circular cup is currently available. An initial biomechanical comparison demonstrated it was the more stable construct when compared to a non-locking metallic dorsal circular plate and K-wire fixation.18 Clinical studies concluded ROM improvements, pain relief, and union rates in line with or better than alternative modes of fixation, and improved perioperative and postoperative radiographic assessment due to the material’s radiolucency.19,20
Foot
The fusion cup system for carpal bones in the hand is also indicated for tarsal bones in the foot.21 Other plates, such as first metatarsophalangeal fusion (MTP) plates and first tarsometatarsal fusion or Lapidus plates have been developed incorporating PEEK. The CoLink™ View Plates provided by In2Bones for MTP and Lapidus fusion offer a titanium plate with a PEEK polymer insert. The PEEK insert can enable transverse lag screw compression across the fusion site plus visibility of the fusion site.
The “Piccolo” MTP and Lapidus plates from CarboFix Orthopedics are made of Invibio’s PEEK-OPTIMA™
Ultra-Reinforced polymer, which combines continuous carbon fibers within a matrix of PEEK for improved strength and fatigue resistance.22 Use of CarboFix
“Piccolo” plates in the foot was described as providing “an unobscured view of the trabeculation across the fusion site in the postoperative period.” 23
Staples and Subtalar Implants
PEEK’s versatility has been evidenced in its incorporation into other device types beyond those already discussed. In2Bones has a large portfolio of PEEK implants for the lower extremity, including a hammertoe implant and plates. Additionally, their product portfolio includes staples (Figure 3) and a subtalar implant (Figure 4). The staples are designed for Akin and Austin Osteotomies and other similar procedures and provide compression on release. As with all other applications, PEEK’s radiolucency is advantageous, and the all-PEEK staples do not raise nickel or metal sensitivity concerns.


Figure 4: O.S.2®-VP Staple PEEK-OPTIMA Natural osteosynthesis staple (left and middle) and X-ray of implanted device images (right) provided courtesy of In2Bones. ©In2Bones.
The PitStop® Subtalar Implant is designed to help facilitate the surgical correction of progressive flatfoot deformity in both pediatric and adult populations. As PEEK is less stiff (softer) than metal and has a similar elasticity to bone, the PEEK material in the implant may lead to increased and long-term patient tolerance.

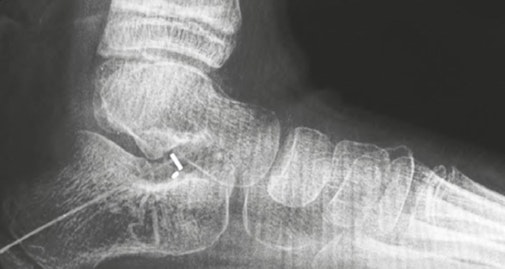
Figure 5: PitStop®Subtalar Implant manufactured from PEEK-OPTIMA™ Natural (left) and X-ray showing its radiolucency (right). Images provided courtesy of In2Bones. ©In2Bones.
Conclusion
Building on PEEK’s clinical efficacy in spine, medical device manufacturers are extending the application of PEEK into implants for hand and foot procedures. Clinical evidence is growing in support of this trend and the material properties of a modulus similar to bone, radiolucency, and inherent lack of metallic response provide benefits to patients and surgeons alike.
Sherri Gambill
Sherri Gambill is currently Trauma Technology Manager at Invibio Biomaterial Solutions™ where she is responsible for product development. Previously, as Business Development Associate she maintained relationships across client organizations as they adopted new biomaterials. Prior to Invibio, Sherri was a Product Development Engineer at DePuy Synthes and BD Opthalmic Systems, where she designed and developed implants and instrumentation for orthopaedic trauma and glaucoma treatment, respectively. In 2006, Sherri received a Bachelor of Science (BS) degree in Bioengineering at the University of Pennsylvania in Philadelphia, PA USA.
You may also be interested in...