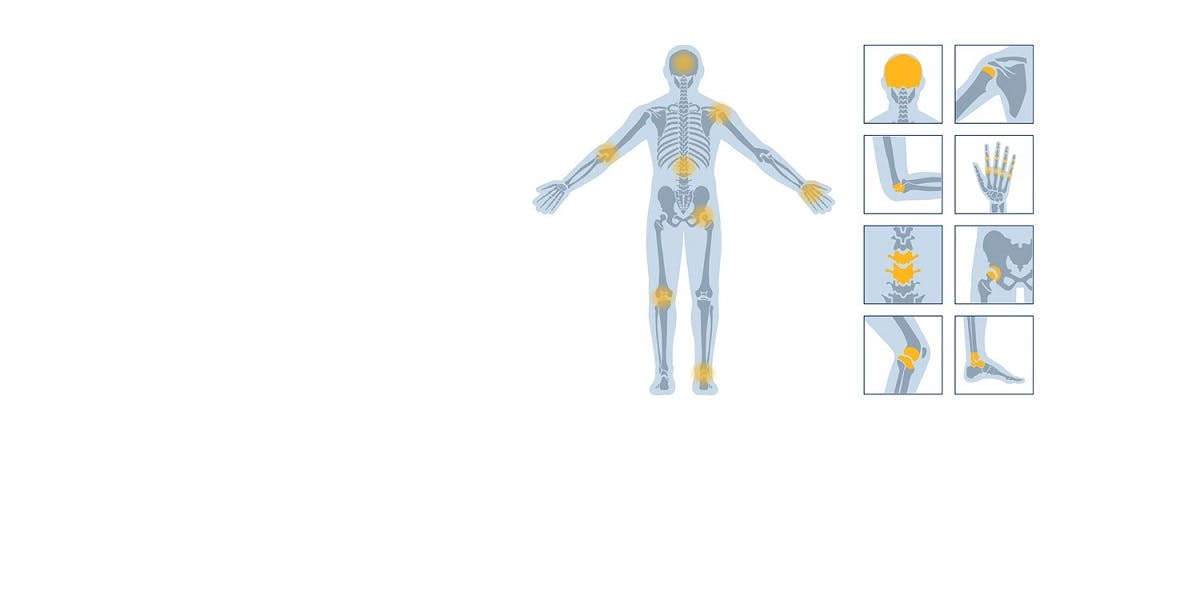
Technology to stimulate innovation
Proven clinical history
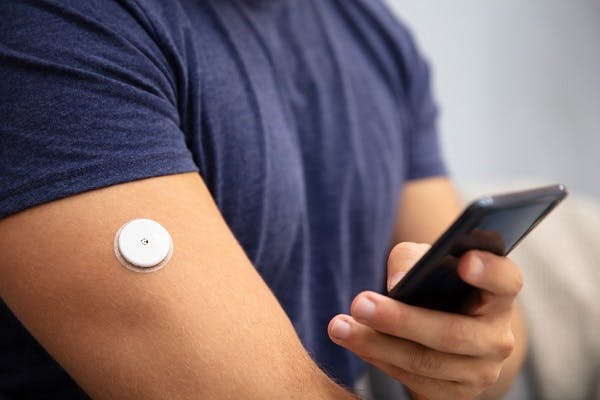
Versatile polymers for a range of next-generation components
PEEK-OPTIMATM polymers have the potential to be used in a broad range of applications including IPG housings & enclosures, neural interfaces, lead and leadless devices and telemetry components
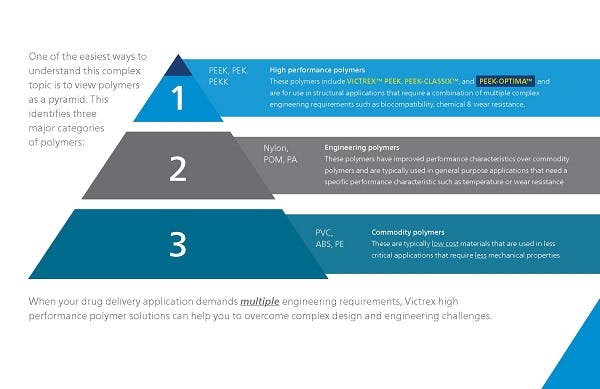
Material selection