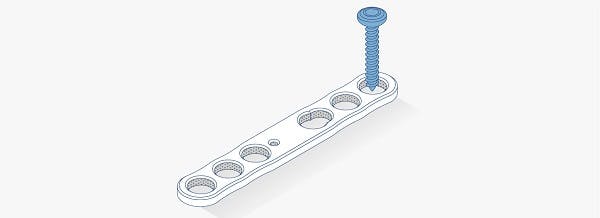
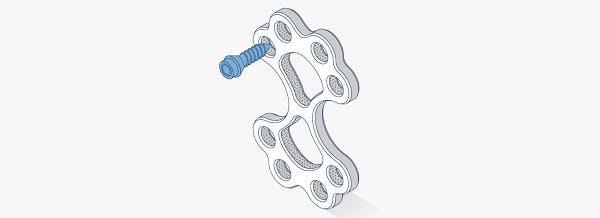
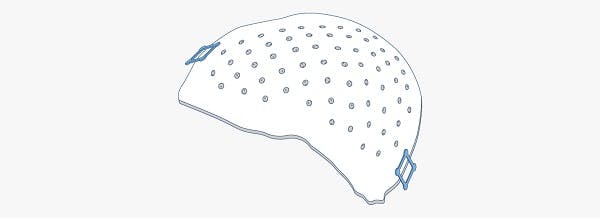
Metal-free, carbon fiber composite fracture fixation solutions
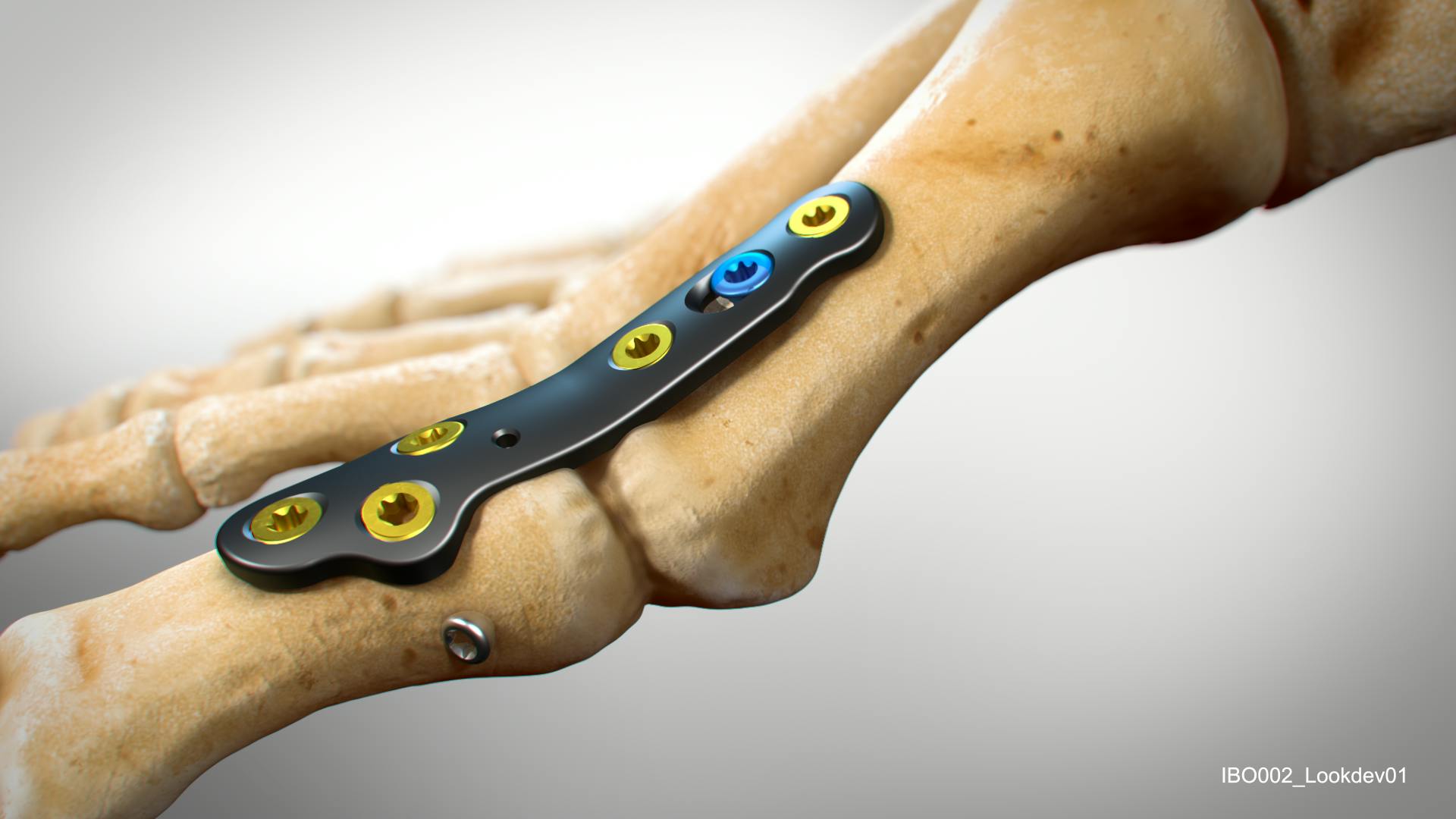
In2Bones & Invibio: collaborate to develop next generation metal-free MTP plates
Discover the benefits of PEEK-OPTIMA Ultra Reinforced polymer in 90 seconds

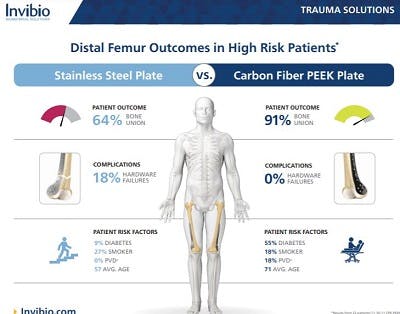


What is PEEK-OPTIMATM Reinforced Polymer?
PEEK-OPTIMA Reinforced polymer consists of short carbon fibers dispersed within the PEEK-OPTIMATM Natural matrix to provide properties well suited for distal radius, proximal humerus and other extremity plates.