A leading choice for spinal fusion & orthopedic devices
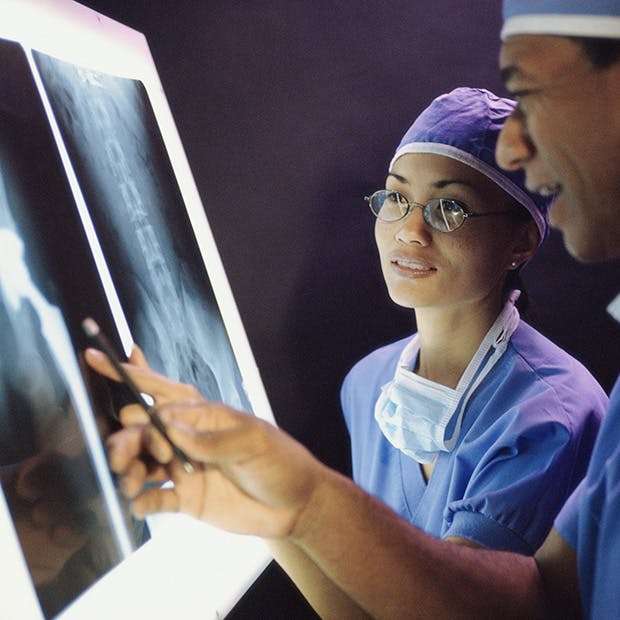
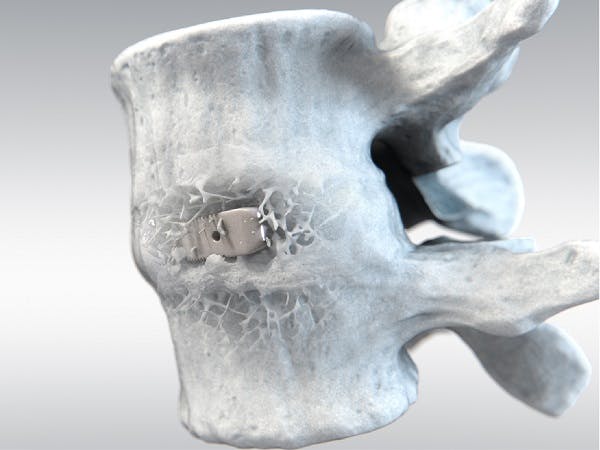



With over twenty years of clinical history, PEEK-OPTIMATM Natural polymer is the first medical-grade PEEK used in spinal fusion surgeries. PEEK is currently the most widely used biomaterial for interbody fusion, accounting for 50% of all devices used1. Clinical studies suggest that PEEK-OPTIMA performs as well as, or better than, interbody fusion devices made of metals or bone, while providing some distinct clinical advantages over competing biomaterials.
In collaboration with leading scientists and research institutions, our biomaterials for orthopedic solutions are proven to deliver improved biomechanics, reduced wear and enhanced bone growth. Innovative orthopedic device companies have chosen our biomaterial solutions for decades.
PEEK-OPTIMA polymers feature mechanical properties that allow reliable arthroscopy applications such as suture retention for knotless suture anchors, tibial sheath and screw systems, and hand, foot & ankle applications.
Interested in evaluating
PEEK-OPTIMATM polymers for your next device?

Beijing Fule and Invibio Biomaterial Solutions co-operation leads to a first for China

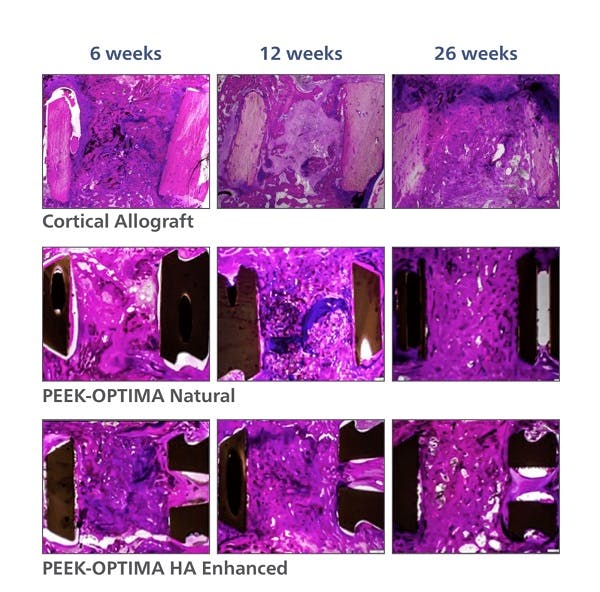
PEEK-OPTIMA HA Enhanced Polymer Ovine Cervical Fusion Study


First Primary Total Knee Arthroplasty with the PEEK-OPTIMA™ Femoral Component
All the benefits of PEEK-OPTIMATM Natural plus Hydroxyapatite (HA) crystals embedded throughout the material, a well-known osteo-conductive material. Unlike interbody fusion devices that are coated, PEEK-OPTIMATM HA Enhanced devices have hydroxyapatite crystals on all surfaces which results in earlier bone on-growth and greater new bone formation on a fully osteoconductive implant. Key benefits include:
A carbon fiber reinforced PEEK polymer compound to increase strength while reducing stress shielding by more closely matching cortical bone stiffness. Short carbon fibers are dispersed within the PEEK-OPTIMATM Natural matrix enhancing mechanical and physical properties for more demanding load-bearing implants and applications. Benefits include3:
An innovative composite formed by continuous carbon fibers dispersed within the PEEK-OPTIMA Natural polymer matrix to enhance mechanical strength and stiffness.
Benefits include:
References for PEEK-OPTIMATM materials
1. Study evaluated the bone ongrowth of PEEK-OPTIMA Natural and PEEK-OPTIMA HA Enhanced in a bone defect model in sheep. Data on file at Invibio. This has not been correlated with human clinical data.
2. Study evaluated the in vivo response to PEEK-OPTIMA Natural, PEEK-OPTIMA HA Enhanced and allograft in a cervical spine fusion model in sheep. Data on file at Invibio. This data has not been correlated with human clinical experience.
3. Supporting information available upon request.
4. Data on file at Invibio Biomaterial Solutions™. Mechanical Benchmark of Carbon Fiber PEEK-OPTIMA™ Ultra-Reinforced vs Ti 6AI-4V Plates undergoing Static and Dynamic Testing per ASTM F382-99 (2008).
References
1. IQVIA, Spinemarket Inc. 2018, Minneapolis, MN

From the invention of PEEK over 40 years ago, Victrex has continually pioneered new PAEK-based polymers, materials and solutions that have transformed markets, delivering global impact in the toughest environments.
We bring transformational & sustainable solutions that address world material challenges every day.