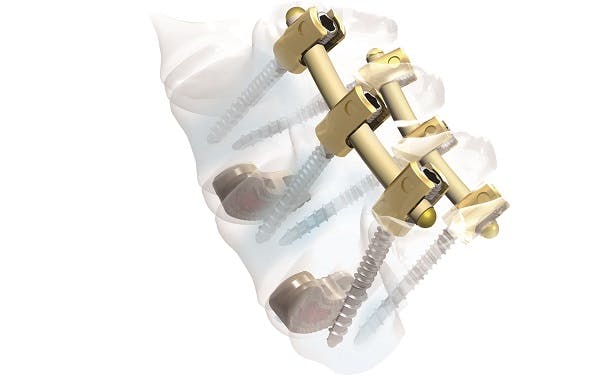
Thornton Cleveleys (UK) – China's largest manufacturer of implants and related surgical instruments for traumatology and spine surgery, Weigao Orthopaedic Device, is offering a spinal stabilisation system called “Tulip”, which uses semi-rigid rods made from PEEK-OPTIMA™ to bridge the gap between rigid and dynamic systems. The system is indicated when one to three levels of the spine, including the lumbosacral region, require posterior pedicle-screw fixation or when fusion surgery is required for a segment of the spine where degenerative disc disease is present.
Weigao chose to work with Invibio based on the company’s track record in PEEK solutions. "Throughout the product development and clinical adoption of our PEEK-OPTIMA semi-rigid rods Invibio was a great help to our company. This includes technical support and testing during the R&D phase," stated Mr. Kui Yang, Vice General Manager of Sales at Weigao Orthopaedic Device. "Invibio invited, for example, overseas clinical experts to China to exchange experience in PEEK-OPTIMA based semi-rigid-rod techniques. In addition multi-centre programme discussions assisted in launching the product and promoting its clinical adoption. We appreciate the value of our relationship with Invibio, and we hope to continue our collaboration."
Two types of the domestic manufactured rods are available: a 6.35mm-diameter round rod, and a 6.35x7.2mm-diameter oval rod. Following previous CFDA (China Food and Drug Administration) approval of other PEEK-OPTIMA-based products, the Tulip system received its approval in 2014 and targets China's Tier-One market for spinal devices as well as Class-Two and Class-Three (top level) hospitals nationally.
PEEK-OPTIMA polymer-based spinal rod components are bridging the treatment gap between rigid and dynamic systems. According to research results presented in Europe the rods are strong enough to restore lumbar spine stability and have much lower failure rates than titanium.1 Spinal rods made from the biocompatible polymer offer numerous advantages, including improved load sharing to encourage fusion,2-4 more physiologic loading at adjacent levels, which may decelerate degeneration 5-6 and reduced stress at the bone-to-screw interface, which may prevent screw pull-out, especially in patients with questionable bone quality.5-6 This improved load sharing, plus clear, artefact-free imaging for easy device placement and monitoring, are important advantages that PEEK-OPTIMA provides over metal rods.
Being non-metallic PEEK-OPTIMA does not produce metal ions when implanted and offers in addition high strength combined with a modulus very similar to that of human bone. The proven implantable material with a 15-year history of successful clinical application has been widely used in the therapeutic treatment of the spine, orthopaedics, arthroscopy and trauma, with PEEK-OPTIMA used in over 9 million implanted devices worldwide.
According to Weigao, surgeons would choose Tulip PEEK rods spinal stabilisation system on the basis of its outstanding value proposition, the advanced polymer-production technology used, and the high reputation accorded to the Weigao brand.
For further information, visit Weigao online at http://en.wegortho.com and Invibio at www.bridgethefusiongap.com.
Caption:
Tulip Spinal stabilisation system uses semi-rigid rods made from PEEK-OPTIMA™ polymer: New therapeutic option in China.
© Weigao Orthopaedic Device Co., Ltd.
REFERENCES
- Schnake KJ, Schaeren S, Jeanneret B: Dynamic stabilization in addition to decompression for lumbar spinal stenosis with degenerative spondylolisthesis. Spine 31:442–449, 2006
- Gornet, Matthew F., et al. (2011). Biomechanical Assessment of a PEEK Rod System for Semi-Rigid Fixation of Lumbar Fusion Constructs. Journal of Biomechanical Engineering, 133, 1-12.
- Moumene, M., et al. (2008). Biomechanical Advantages of Expedium PEEK Rods.
- Galbusera F., et al. (2010). Rigid and Flexible Spinal Stabilization Devices: A Biomechanical Comparison. Medical Engineering & Physics, 33, 490-496.
- Ormond, DR., et al. “Polyetheretherketone (PEEK) rods in lumbar spine degenerative disease: a case series. J Spinal Disord Tec, 12(8), 693-701.
- De Iure, F., et al. (2012). Posterior lumbar fusion by PEEK rods in degenerative spine: preliminary report on 30 cases. Eur Spine J, 21(1), S50-S54.
About Weigao
Weigao Orthopaedic Device Co., Ltd. is China's largest manufacturer of implants and related surgical instruments for traumatology and spine surgery. In China, we work with research institutions in the development of innovative technology, assist top hospitals offering effective treatment solutions, and restore patients to health. We are dedicated to providing our excellent products and services to surgeons, hospitals and patients worldwide.
About Invibio Biomaterial Solutions
Invibio™, a Victrex plc company, is a global leader in providing high performance biomaterial solutions to medical device manufacturers. The company provides PEEK-OPTIMA™ polymers, advanced technical research and support and manufacturing of components for spine, trauma and orthopaedic medical segments for the development of long implantable medical devices. Today, Invibio’s PEEK-OPTIMA™ polymers are used in more than five million implanted devices worldwide.
About Victrex plc
Victrex, headquartered in the UK, is an innovative world leader in high performance polymer solutions focused on the Aerospace, Automotive, Electronics, Energy and Medical markets. Every day, millions of people rely on products or applications which contain our polymers, from smartphones, airplanes and cars to oil & gas operations and medical devices. With over 35 years’ experience, we are investing in technical excellence to deliver leading edge solutions to our customers and our markets, and to drive value for our shareholders. Find out more at www.victrexplc.com
Contacts
Invibio Biomaterial Solutions
Carina Wang, Invibio Asia Marketing Manager
cwang@invibio.com
phone: +86-10-57379295
Victrex media contact
Beate Sauer, PR & Marketing Communications Manager
bsauer@victrex.com
phone: +49 61 92 / 96 49 19
Group media or Investor Relations enquiries
Andrew Hanson, Head of Investor Relations & Communications
ahanson@victrex.com
phone: +44 12538 98121