References
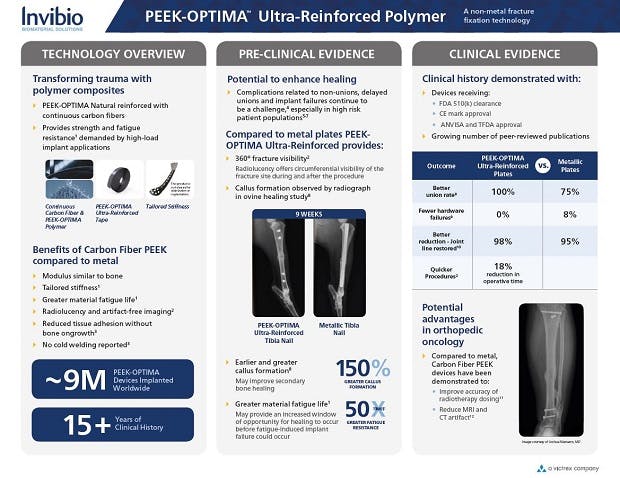
1.Data on file at Invibio Biomaterial Solutions. Mechanical Benchmark of Carbon Fiber PEEK-OPTIMA™ Ultra-Reinforced vs Ti 6Al-4V Plates undergoing Static and Dynamic Testing per ASTM F382-99 (2008).
2.Ziran BH, Mansour J, Jahangir AA, Min W. Efficacy of Carbon Fiber-Reinforced PEEK Femoral Nail Preliminary Results. Poster #68. Presented at OTA 2017.
3.Allison DC, Menendez LR. Carbon fiber fixation in oncologic bone surgery. Presented at MSTS Meeting 2013. Carbon Fiber Intramedullary Nail Fixation in Troublesome Long Bone Fractures and Nonunions in Cedars-Sinai Advances in Orthopaedics Newsletter, Spring 2014.
4.Zimel MN, Hwang S, Riedel ER Healy JH. Carbon fiber intramedullary nails reduce artifact in postoperative advanced imaging. Skeletal Radiol. 2015 Sep;44(9):1317-25.
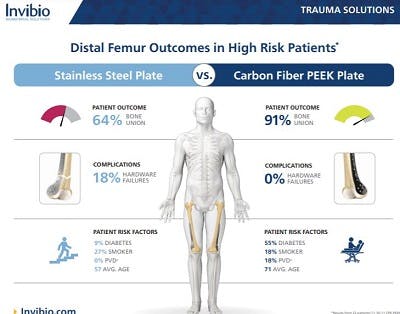
5.Zura R, Xiong Z, Einhorn T, Watson JT, Ostrum RF, Prayson MJ, Della Rocca GJ, Mehta S, McKinley T, Wang Z, Steen RG. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg. 2016 Nov 16;151(11):e162775. doi: 10.1001/jamasurg.2016.2775. Epub 2016 Nov 16. PMID: 27603155.
6.Sathiyakumar V, Thakore RV, Greenberg SE, Whiting PS, Molina CS, Obremskey WT, Sethi MK. Adverse Events in Orthopaedics: Is Trauma More Risky? An Analysis of the NSQIP Data. J Orthop Trauma. 2015 Jul;29(7):337-41. doi: 10.1097/BOT.0000000000000293. PMID: 26091531.